Introduction
Recently, the European Leukemia Net (ELN) recommended cytoreduction in patients (pts) with Polycythemia Vera (PV) at low risk (LR) (age <60 yrs & no previous thromboses) but carrying additional criteria for therapy start (CTS) ( Marchetti M et al, Lancet Haematol 2022). Little is known about: 1] efficacy of hydroxyurea (HU) in LR pts, high risk due to age >60 yrs (HR-AGE) or high risk due to previous thrombosis regardless of age (HR-THRO); 2] impact of CTS on thrombotic risk.
Aims
In a large cohort of HU-treated PV pts, we investigated if thrombotic risk was influenced by: 1] conventional risk factors for thrombosis; 2] ELN-defined complete response (CR) at 12 mos; 3] presence of CTS.
Methods
After IRB approval, data of 961 WHO2016-defined PV pts were retrospectively collected by 21 Hematology Centers. In April 2023, an update was performed in 10 participating Centers with specific focus on CTS at diagnosis and HU start; 504 HU-treated pts resulted eligible for this analysis. Therapy was at discretion of the treating hematologist. Pts were followed until death or data cut-off date.
CTS evaluated for association with thrombosis included: persistent/progressive leukocytosis (100% increase if white blood cells (WBC) <10 x10 9/L or 50% increase if WBC>10 or WBC>15 at diagnosis and HU start); extreme persistent thrombocytosis (>1000 x10 9/L platelets (PLT) at diagnosis and HU start); progressive splenomegaly (increase of >5 cm from diagnosis); inadequate hematocrit (Hct) control (>6 PHL/yr; HCT >53% at diagnosis and HU start; PHL intolerance); uncontrolled cardiovascular risk factors (CVRF). Symptoms were present as the only ELN criterion in 40 patients, of whom none had thrombosis; therefore, they were not included in the analysis.
Results
504 pts received HU: 81 pts were at LR and 423 (83.9%) were at HR (HR-AGE, no. 290, 57.5%; HR-THRO, no. 133, 26.4%). Median time on HU was 11.6 yrs (0.1-23.1).
During HU, 53 pts had 69 thromboses (35 arterial) for an incidence rate of thrombosis (IR-thro) of 1.5 %p-y, comparably in LR and HR ( Table 1). Notably, IR-thro was equal in LR and HR-AGE (p=0.94), but significantly higher in HR-THRO pts (p=0.005 vs LR and p=0.009 vs HR-AGE).
At 12 months, CR was achieved by 8.2%, 23% and 25% of LR, HR-AGE and HR-THRO pts, respectively (LR vs HR-AGE and HR-THRO, p<0.001 and p=0.003).
Thrombosis-free survival (TFS) by landmark analysis at 12 months was significantly better in CR pts (p=0.01). Specifically, best TFS was observed in LR and HR-AGE pts with CR, while HR-THRO pts without CR had the worst TFR (p=0.009).
CTS were observed in 255 (50.6%) pts: 48 LR (59.3%), 139 HR-AGE (47.9%) and 68 HR-THRO (51.1%). In the remaining LR pts, HU was triggered by mild thrombocytosis (<1000 x10 9/L) and/or mild leukocytosis (<15 x10 9/L) and/or mild splenomegaly (<5 cm from costal margin) and/or persistent systemic symptoms.
CTS were associated to significantly increased of IR-thro in the overall cohort (p<0.001), in LR pts (p=0.04), in HR-AGE pts (p=0.02) and in HR-THRO pts (p=0.03) ( Table 1) . The impact of CTS was confirmed in the overall cohort both for arterial (1.30 vs 0.44 %p-y, p=0.01) and venous thrombosis (1.37 vs 0.44 %p-y, p=0.007).
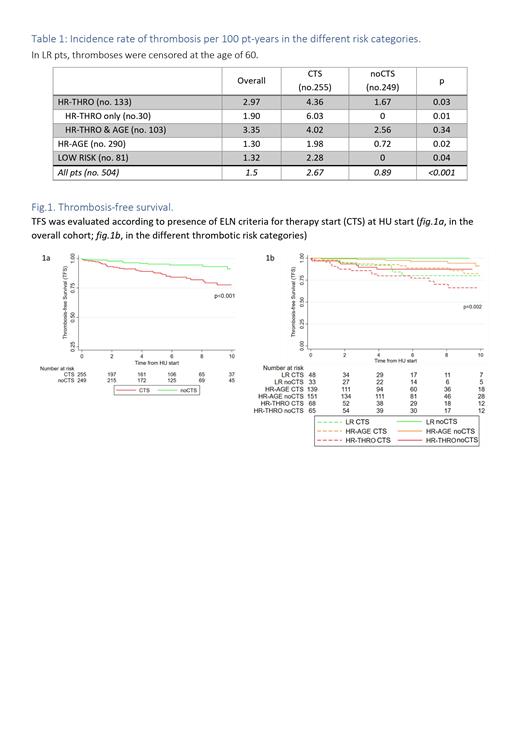
TFS at 5 yrs was 87% and 95% in pts with or without CTS (p<0.001) ( Fig.1a). In presence of CTS, TFS of LR and HR-AGE pts were comparable to HR-THRO pts without CTS ( Fig. 1b).
In multivariate Cox analysis considering age>60 yrs, previous thrombosis, CTS, and CR to HU at 12 months, CTS and previous thrombosis remained associated with thrombotic risk (HR: 2.7, 95% CI: 1.4-5, p =0.002; HR: 2.4, 95% CI: 1.4-4.2, p =0.001).
Finally, in 59 additional LR pts untreated with HU, IR-thro was 0.78 in absence of CTS, and 1.57 %p-y in presence of such criteria.
Conclusions
Albeit retrospective, this study shows for the first time that ELN criteria for therapy start including splenomegaly, leukocytosis, thrombocytosis, uncontrolled Hct and CVRF, identified an increased risk phenotype either in LR and HR pts treated with HU. This was confirmed also in HU-untreated pts.
Also, lack of CR at 12 months was detrimental on TFS particularly in HR-THRO pts, and less significantly than CTS.
These findings, that complement data from the Majic-PV trial ( Harrison CN et al, J Clin Oncol 2023), dictate better management of HU therapy, and call for greater attention to CTS and their integration into prognostic models that go beyond the two categories related only to age and thrombotic history.
Disclosures
Palandri:Novartis, BMS, Celgene, GSK, Amgen, AbbVie, Karyopharm, AOP, Sierra Oncology, Janssen: Consultancy, Honoraria. Elli:Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Latagliata:Janssen: Honoraria; Novartis: Honoraria; BMS: Honoraria; Celgene: Honoraria. Cavazzini:Novartis: Honoraria; Pfizer: Honoraria; Incyte: Honoraria. Polverelli:Abbvie: Honoraria; GSK: Honoraria; BMS: Honoraria; Novartis: Honoraria. Abruzzese:Takeda: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cavo:GlaxoSmithKline: Honoraria; Adaptive: Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene/Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Takeda: Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Honoraria; Roche: Honoraria. Palumbo:Incyte: Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; BeiGene: Honoraria; Janssen: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; AOP: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Heidel:Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; CTI: Consultancy, Honoraria, Research Funding; Kartos: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding. Breccia:BMS: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; AOP: Honoraria; AbbVie: Honoraria; Novartis: Honoraria. De Stefano:Alexion: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Grifols: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; SOBI: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal